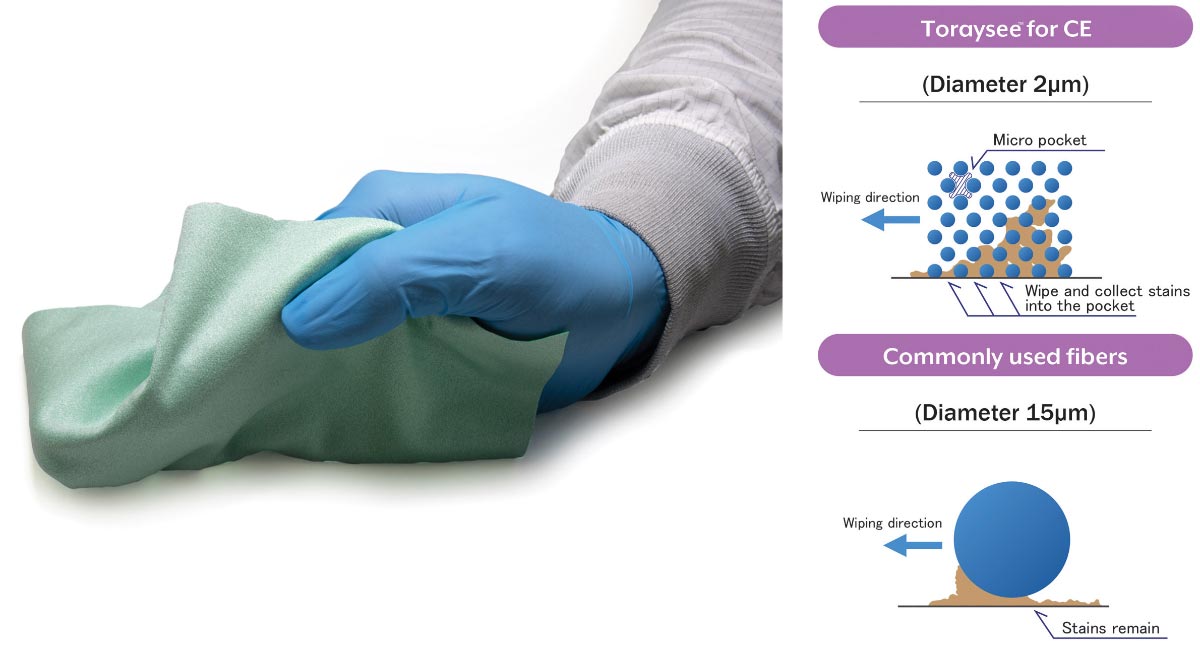
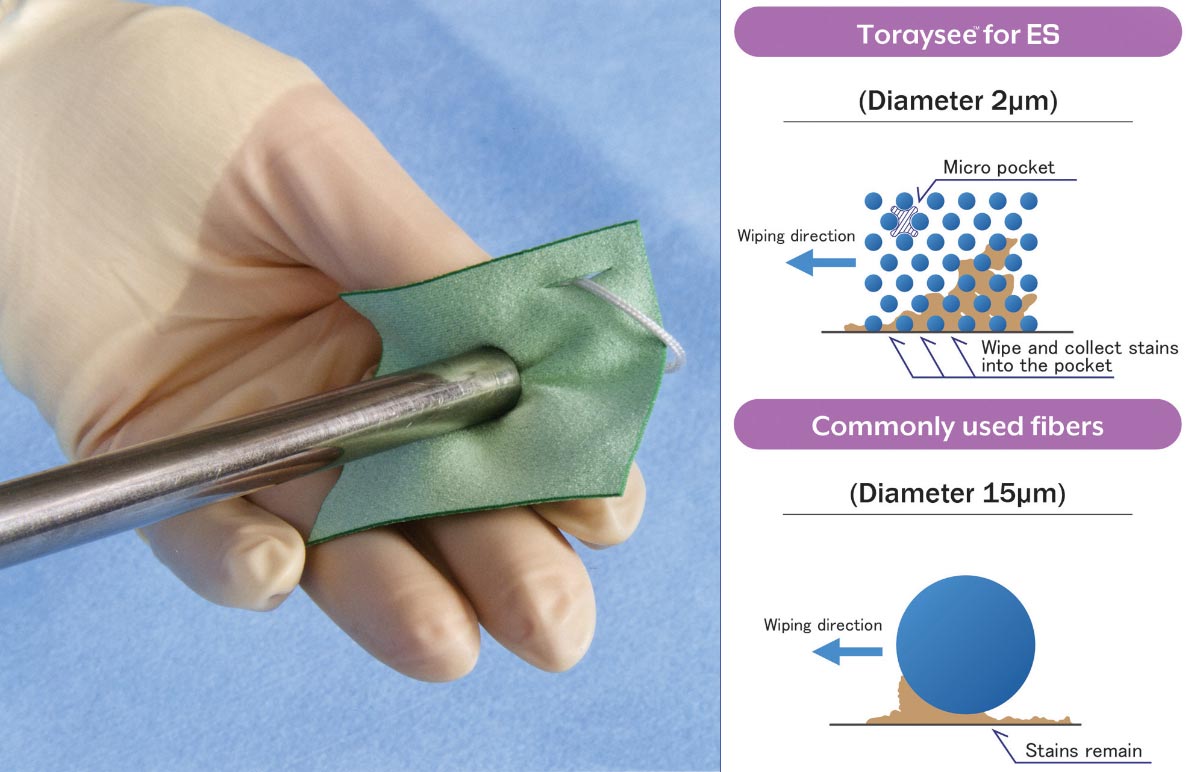
Toyolac®’s lower molding temperatures and lower pressure requirements compared to other resin materials is proving to help increase production capacity and turnaround time for Covid-19 related medical components. With operating cylinder temperatures ranging from 210°C to 260°C, Toyolac® provides smoother resin flow, increased cycle times with decreased part flash. This enhanced flowability provides increased part cavitation while providing better molding of thin-walled part features with less tooling wear and longer mold life.
New York, NY: Toyolac®’s lower molding temperatures and lower pressure requirements compared to other resin materials is proving to help increase production capacity and turnaround time for Covid-19 related medical components. With operating cylinder temperatures ranging from 210°C to 260°C, Toyolac® provides smoother resin flow, increased cycle times with decreased part flash. This enhanced flowability provides increased part cavitation while providing better molding of thin-walled part features with less tooling wear and longer mold life.
Toyolac® has a stable quality and enables long-term adoption because of Toray’s no change “Production Methods”, no change “Formulations” and no change “Test Methods/Equipment” policy ensuring long term stability for manufacturers and regulatory requirements. Toyolac® is a well-balanced resin and a FDA Drug Master File registered material. It is USP Class 6 tested and meets ISO 10993 for biocompatibility. Specific tests include -4 for blood interactions, -5 for in vitro cytoxicity, -10 for irritation and skin sensitization and -11 for systemic cytotoxicity. (Toyolac® does not contain bisphenol-A, an industrial chemical commonly used to make plastics which through exposure can cause health problems.)
For more information about TOYOLAC® Transparent ABS Resin, call 612-979-0613 to learn more. Or Email: [email protected] www.toray.us Toray Industries, Inc., 461 Fifth Ave, 9th Fl., New York NY 10017
About Toray
Toray Industries, Inc., is a world class, global, integrated, chemical industry group with businesses in 26 countries and markets worldwide. In 2018, Toray's sales exceeded $22 Billion and employed over 45,000 people worldwide. Toray combines nanotechnology with its core technologies of organic synthetic chemistry, polymer chemistry and biotechnology. These advanced basic materials technologies are applied to develop products in the chemicals, plastics, films, fibers, textiles, composites, pharmaceuticals and medical market segments. Toray's "Innovation by Chemistry" is applied in the medical market to create unique materials that benefit cutting edge products and provide more effective treatments at lower cost and less risk than current products.