Toray Industries, Inc., announced today that it has developed fast-acting antiviral particles.
Conventional disinfection with antiseptic solutions and other chemicals is effective and fast- acting on viral infections. The downside, however, is short volatilization, necessitating regular disinfections. While non-volatile metal-based antivirals offer generally lasting protection, the issue is that many of them take at least an hour to deactivate 99.9% of viruses.
Tokyo, Japan, May 26, 2022 – Toray Industries, Inc., announced today that it has developed fast-acting antiviral particles.
Conventional disinfection with antiseptic solutions and other chemicals is effective and fast- acting on viral infections. The downside, however, is short volatilization, necessitating regular disinfections. While non-volatile metal-based antivirals offer generally lasting protection, the issue is that many of them take at least an hour to deactivate 99.9% of viruses.
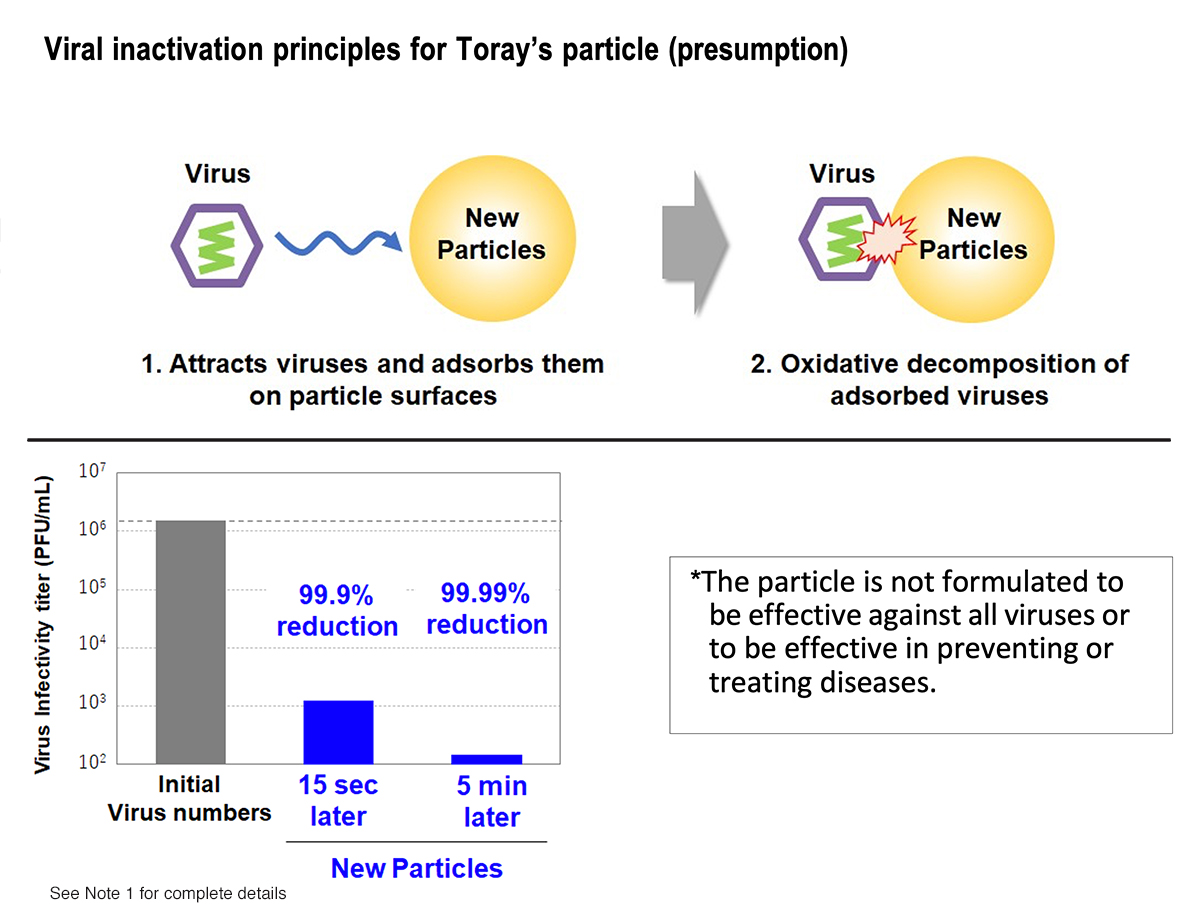
Toray responded to that situation by developing antiviral particles that deactivate 99.9% or more of SARS-CoV-2 virus (the cause of COVID-19) strains in just 15 seconds and 99.99% or more of the strains within 5 minutes. The company achieved this by adding virus adsorption and oxidative degradation capabilities to cerium oxide particles through proprietary synthesis and surface treatment techniques. Toray drew on functional particle design, synthesis, and surface control technologies that it has developed over the years. The new particles deactivate viruses around 100 times faster than conventional metal-based antiviral agents. They are thus among the world’s quickest deactivation delivery vehicles.
Another benefit is that the particles do not volatilize and are lasting protection. That is because they do not use the virus deactivation principle of slow releases with drugs, metal ions, or other active ingredients. The particles also offer excellent safety (see note 2) and resist discoloration and corrosion.
Prospective media for the particles include building materials, paints, and packaging materials. They could thus be deployed in diverse products, including in public spaces in which numerous people might gather, necessitating measures to safeguard from virus infection. Other targets could be public transportation facilities, restaurants, medical and eldercare facilities, interior walls and railings at schools, and regular appliances and food packaging.
Antiviral particles could coat or be kneaded into diverse items. These could include non-woven fabrics for Toray’s masks and medical gowns, air filters, car seats, and other products that could benefit from these particles to prevent droplet and contact infections. Toray will gradually roll out test samples of the particles to customers.
The creation of these particles stemmed partly from a joint research effort with Professor Satoshi Taharaguchi of the School of Veterinary Medicine at Azabu University under the New Energy and Industrial Technology Development Organization’s Feasibility Study Program on Materials and Biotechnology.
Toray is leveraging its core technologies of synthetic organic and polymer chemistry, biotechnology, and nanotechnology to research and develop advanced materials that transform societies. The company will keep pushing ahead with its commitment to innovating ideas, technologies, and products that deliver new value, accelerating R&D to safeguard societies from viral infection and contribute to healthier living.
Benefit summary
2. Prospective applications
Notes
2. Particle safety testing results
Items: Acute toxicity (oral) – Results: GHS class 4 or higher
About Toray
View website