Qosina outlines factors to consider in the selection process as per the ASTM E3051-16 Standard Guide for Specification, Design, Verification, and Application of Single-Use Systems in Pharmaceutical and Biopharmaceutical Manufacturing, to help customers choose a bag chamber that ensures optimal performance for their application and user requirements.

There are many factors to consider when selecting an appropriate bag chamber for a single-use system to support your bioprocess. The task can seem overwhelming, so it is important to lean on stringent procedures to ensure a successful outcome.
ASTM E3051-16 Standard Guide for Specification, Design, Verification, and Application of Single-Use Systems in Pharmaceutical and Biopharmaceutical Manufacturing describes a rigorous process for implementation of single-use systems, which is intended to conform to regulatory requirements following a risk-based approach. The basic elements of E3051 can be applied to bag chamber selection for use in a single-use system. Below we provide an example of the following concepts of E3051 for bag chamber selection.
The first step is to comprehensively define your user requirements: specific requirements should be identified and provide the basis of detailed specification, design and verification of the SUS.
Typical bag chamber-specific user requirements would include:
- Integrity risk assessment
- Particulate risk assessment
- Intended chamber contents
- Liquid volume
- Application (liquid or frozen, storage, shipping, mixing, etc.)
- Temperature range
- Chemical compatibility
- Duration of application
- Light sensitivity
- Barrier requirements (oxygen, carbon dioxide, water vapor, etc.)
- Drainability
- Handling considerations
According to E3051, the specific requirements relative to product quality and patient safety should be based upon the following:
- Product knowledge and understanding refers to the process fluid itself and can help identify risks to the product or process fluid from the system, or risks to the system from the product or process fluid.
- Process knowledge and understanding help identify risks that the process can bring to the bag chamber or that failure of the bag chamber can bring to the process.
- Materials knowledge and understanding will help guide the bag chamber selection since bag chambers are available with different construction materials.
- Supply chain knowledge and understanding can help you identify risks to supply interruption.
- Knowledge of the regulatory requirements for the application will help avoid selecting the wrong bag chamber.
- Many companies have defined quality requirements specific to different applications or as blanket requirements for the selection of single-use system components. A summary of these requirements is useful to bag chamber selection.
Qosina offers bag chambers made from different materials, in many volumes, port configurations and types. We maintain an extensive ISO 13485 controlled database of relevant product attributes to help in the selection of components that meet your needs, including bag chambers.
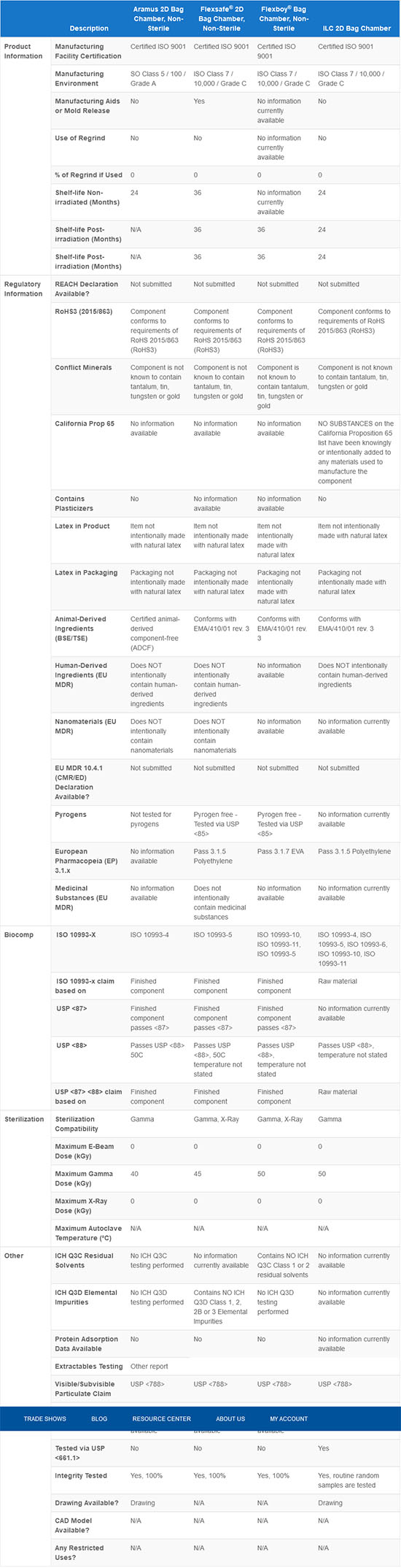
The information in the above table is true as of the writing of this content. Qosina is continuously improving our database with the most complete and up-to-date information. If you require specific information that is not available, please contact Qosina for assistance.
By comparing your user requirements against the specifications maintained in our systems, you can ensure that you make the right choice when selecting bag chambers
View website