Patricia Frey, Director of Technology and Development and Ian Christopher, Research & Development and Product Manager for Scapa Healthcare in the US, shared their thoughts about how Scapa Healthcare is helping its customers create innovative fixation solutions to support the development of new monitoring devices.
The continuous glucose monitoring market is rapidly growing due to the increasing prevalence of diabetes coupled with recent trends, including digital therapeutics and wearables. Patricia Frey, Director of Technology and Development and Ian Christopher, Research & Development and Product Manager for Scapa Healthcare in the US, shared their thoughts about how Scapa Healthcare is helping its customers create innovative fixation solutions to support the development of new monitoring devices.
Can you each briefly explain your role at Scapa Healthcare?
Patricia Frey: My role as Director of Technology and Development is to work with our customers on specific applications to deliver custom products for medical device and skin contact applications. I work closely with our application engineers to creatively develop new product concepts to transform customer user requirements into materials and converted components for commercially viable products.
Ian Christopher: I am a Research & Development and Product Manager for Scapa’s Custom Adhesive Coating Technology Center in Windsor, CT, where we produce solvent-based, pressure-sensitive adhesives and silicone gel adhesives. I have been with Scapa for close to 18 years and have spent most of that time working with specialized medical pressure-sensitive adhesive product designs.
How does a glucose monitoring device work?
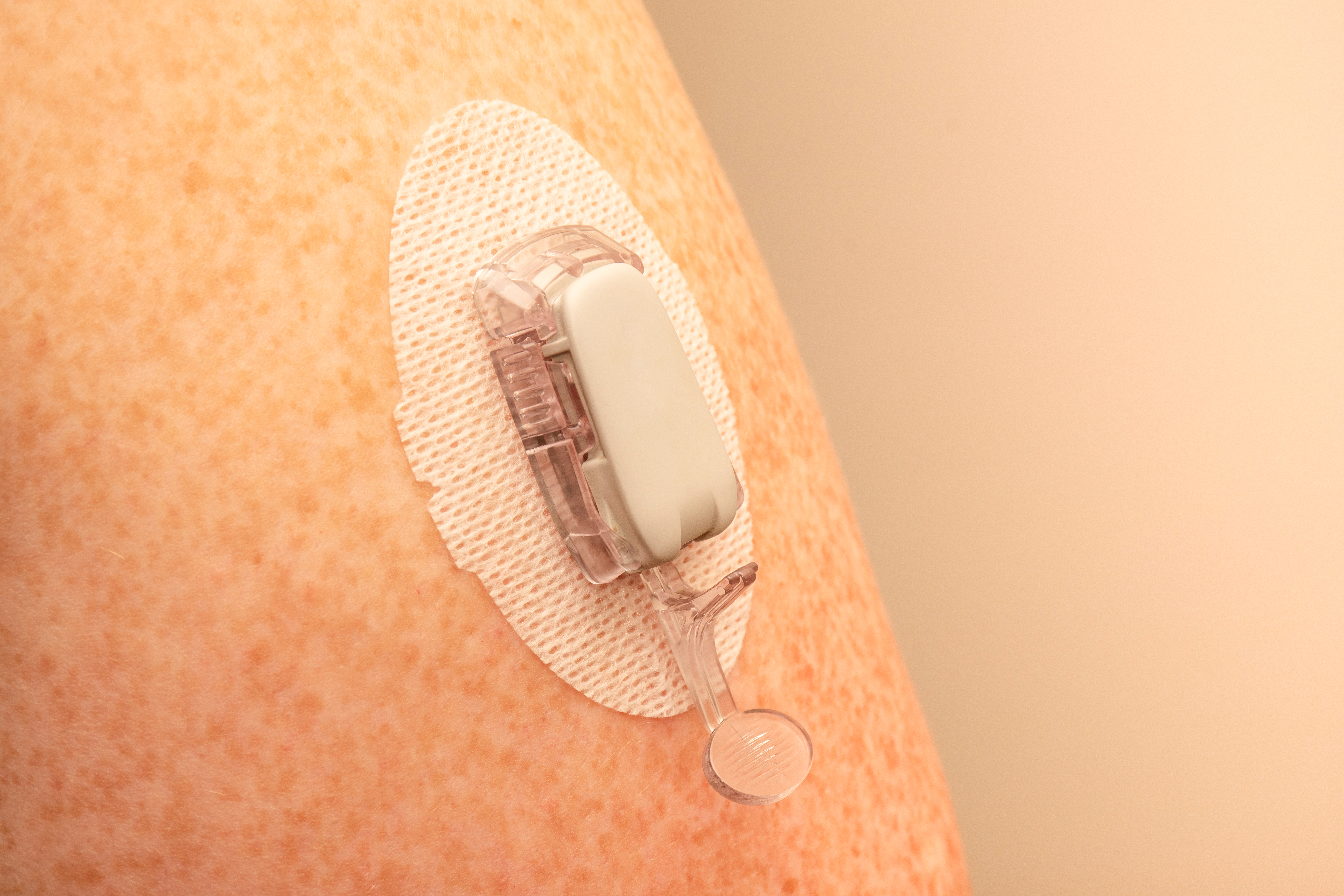
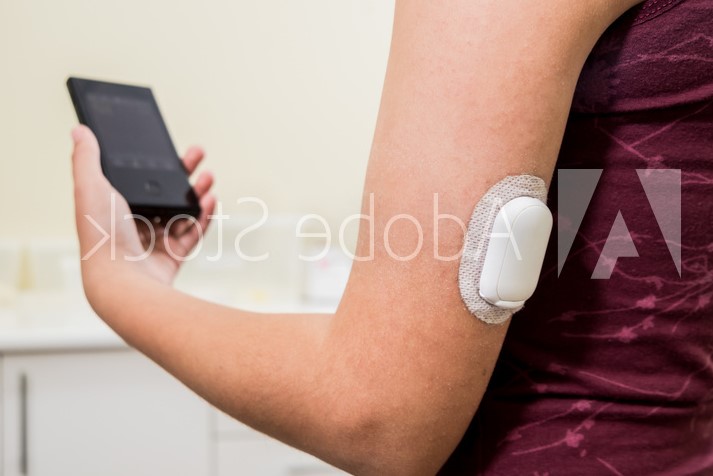
Continuous glucose monitoring (CGM) devices provide an effective method for measuring blood glucose levels in real-time. The data recorded by CGM devices is typically transmitted through a wireless communications network to help keep track of a patient’s specific blood glucose levels over a designated period based on their physical activity, food consumption, medications, and more.
How do you see the glucose monitoring device market growing over time?
Recently, there has been strong growth in the market for innovative skin-based CGM devices. Several ongoing developments are driving this demand, including wearables, slimmer, flatter, more lightweight device designs with longer wear times, to help improve patients experience managing their diabetes. Additional factors include a growing geriatric population prone to diabetes, rising awareness about preventive diabetes care, recent FDA approvals of new CGM systems and suite of digital health tools for improved diabetes management.
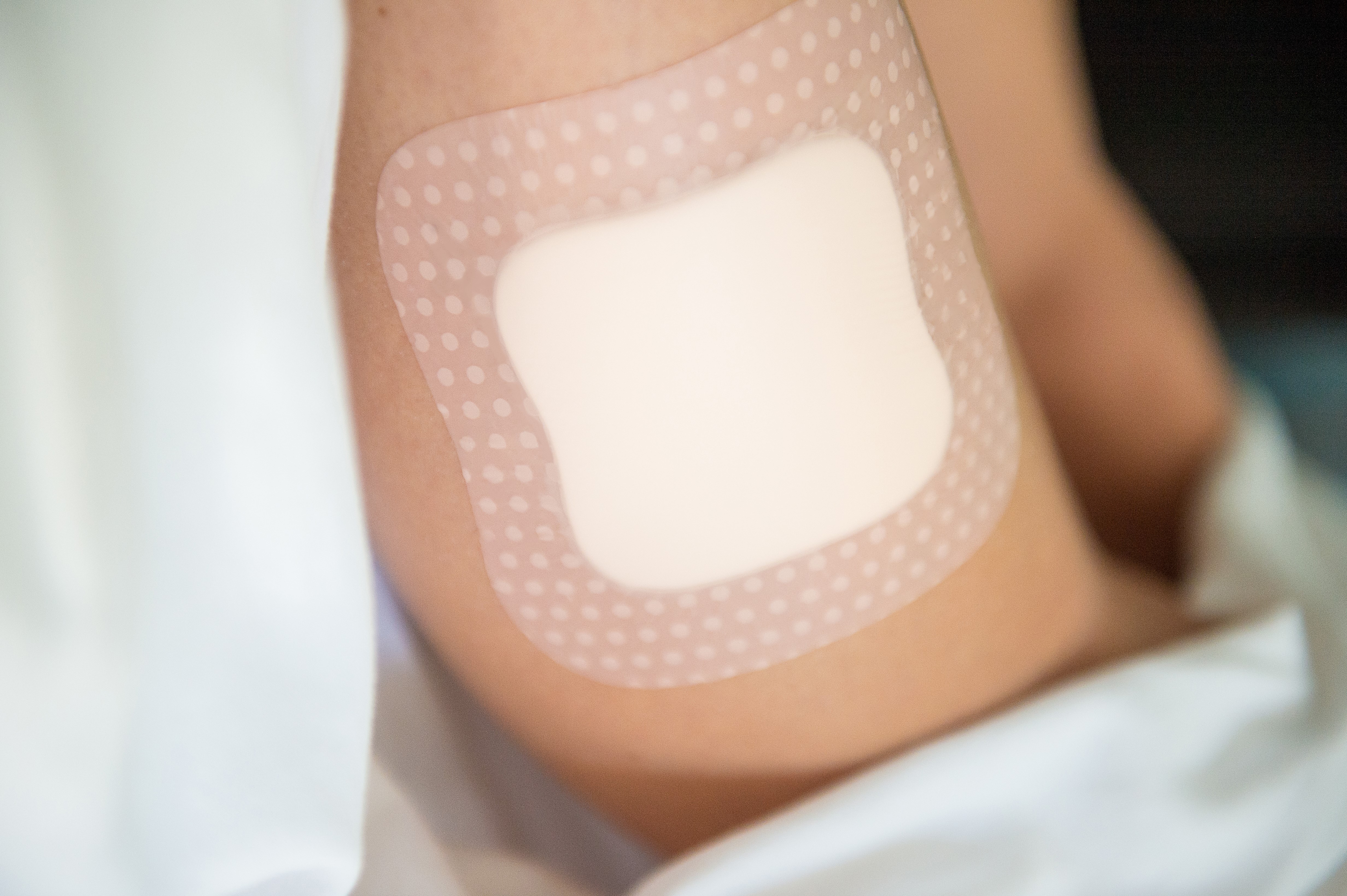
Regarding medical grade skin contact adhesives for CGM devices, the standard keeps getting higher in terms of improved wear time, easier removal, and more consistent wear on the body.
How does Scapa Healthcare strategically support its partners in developing the optimal medical-grade skin contact adhesives to meet their CGM device needs?
Scapa Healthcare strategically supports its customers by first looking at their unique application, agreeing on the specific requirements for the device and skin contact needs, and creating different constructions of biocompatible substrates and adhesives. We then perform testing to show comparisons between the options and do a user study to get real-world data. We model the tests to align with the size, shape, and weight of the device. This helps our customers know they are getting the best solution that meets their custom device designs.
What properties and circumstances need to be considered when formulating an adhesive for a glucose monitoring device? What are you looking for in a successful product test?
To determine what type of adhesive will work best, we initially need to understand several factors. For example, the intended wear time/how long it will be on the patient, and the target demographic for the device, e.g., is it designed for those with an active lifestyle or someone who has limited to no mobility? The less activity, the gentler the adhesive, the more active, the more aggressive the adhesive. We also need to understand where the CGM device will be placed on the body (i.e., abdomen vs. arm) to determine the optimal adhesive choice and formulation.
In our product testing, we are looking for how well the adhesive holds the device, which construction will stay on the body for the required amount of time with no partial removal, and an adhesive that can be removed with the least amount of pain possible. In addition to actual user studies, we also use industry standard testing methods, including American Society for Testing and Materials (ASTM) or Pressure Sensitive Tape Council (PSTC), for measuring pressure-sensitive adhesives.
How have you seen CGM devices, particularly the associated adhesives, evolve over the years?
CGM devices have essentially evolved from a finger prick to a wearable. Other ways the devices themselves have evolved over the years include the fact that wear times are getting longer, and devices are getting smaller and lighter in weight.
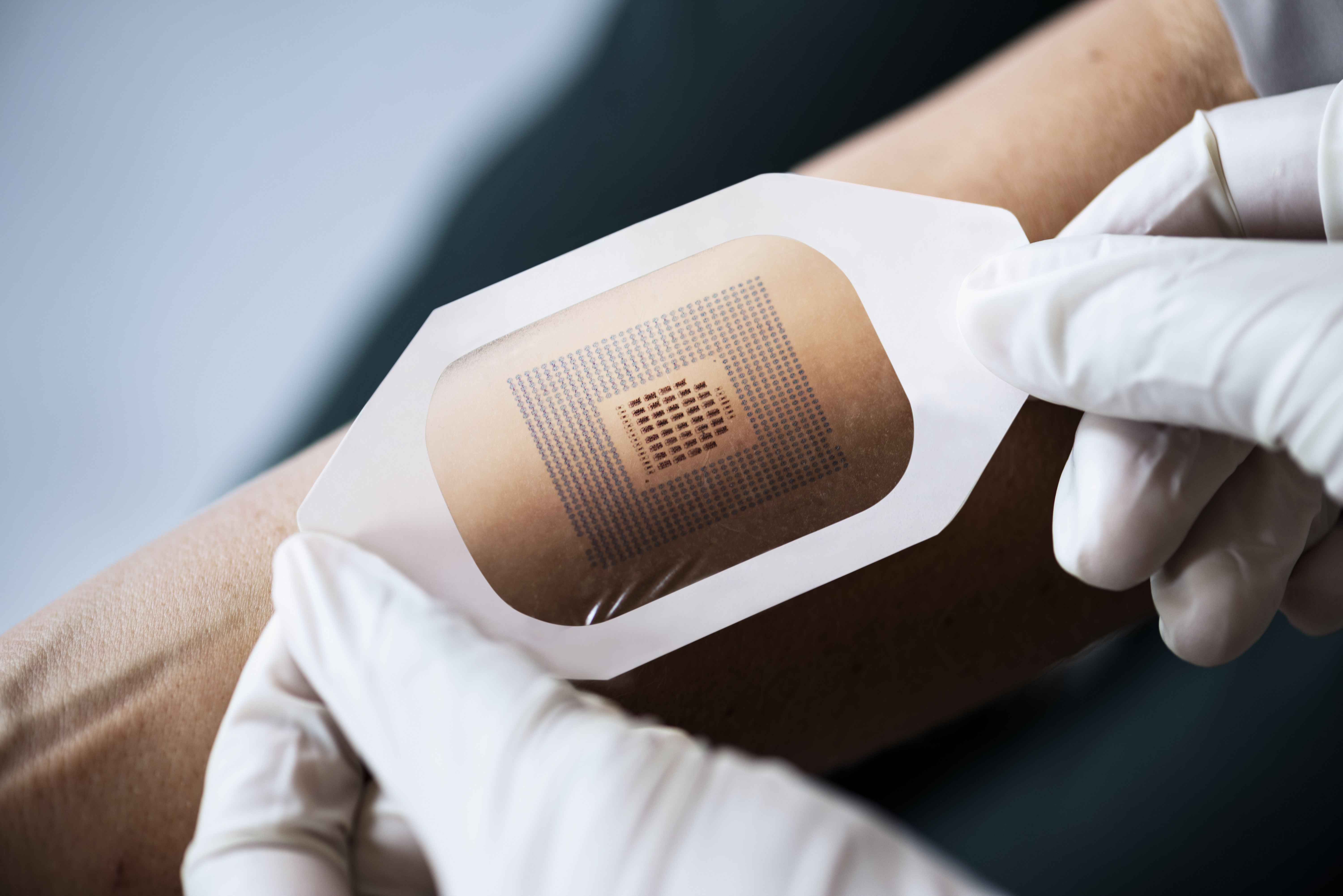
Adhesive technology changes come down to the combination of the adhesive and the materials being used in the skin contact layers. Some of these combinations are changing because the device may have a nonwoven polyester as a skin contact layer with an acrylic adhesive. Others may need to be water-resistant, requiring a polyurethane film or foam.
What are some of Scapa’s unique value propositions in this market?
Scapa Healthcare works closely with its customers to fully understand their unique CGM device requirements and customize the substrates that better fit their specific product claims. Scapa Healthcare stands out in this space as we have the ability to work with a broad range of materials and adhesives to create unique combinations for our customers to try. We have the flexibility to fully customize solutions to meet their individual and specific needs versus making the customers squeeze into certain preexisting products. In addition, our team of experts also takes customer requirements from concept to formulation development to raw material sourcing and prototyping to full-scale production of medical-grade materials. For example, we are able to suggest specific design features or recommend release materials to enhance the user experience.
We are also fully integrated with other Scapa sites to convert, package and deliver the optimal solutions to our customers. Our expertise can help customers weigh the aspects that must be considered from the regulatory point of view as well as internal and external supply chain capabilities
View website