The investment of innovation, development, production, and successful integration of vascular access devices are critical to advancing patient care. These products are designed to help healthcare professionals gain safe access to the cardiovascular system through a variety of devices allowing them to monitor and treat a wide range of clinical conditions.
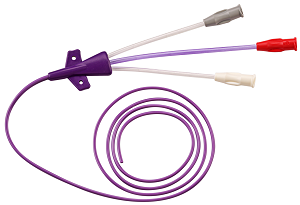 Flexan has been deeply involved in the development and production of catheters for a variety of vascular access purposes. From catheter design for manufacturability (DFM) to developing proprietary manufacturing processes, we have worked with our partners to create products that are safe and effective for medical professionals and their patients.
Flexan has been deeply involved in the development and production of catheters for a variety of vascular access purposes. From catheter design for manufacturability (DFM) to developing proprietary manufacturing processes, we have worked with our partners to create products that are safe and effective for medical professionals and their patients.
There are numerous technical challenges associated with developing vascular access products that meet the stringent requirements for efficacy, outcome improvement, and patient safety. Working with a proven manufacturing partner like Flexan will help identify and overcome those challenges.
What is Vascular Access Product Development and Why Is It Important?
Vascular access product development is the process of designing, testing, and producing medical devices that provide access to a patient’s vasculature. These devices allow healthcare professionals to monitor and administer fluids, deliver medications, and or repair vascular structures for patients. Vascular access product development is an important branch of medical device engineering and is used to improve the safety, efficacy, and accuracy of patient care.
Vascular Access Product Development Challenges
Vascular access is an essential component of modern clinical practice and thus offers an opportunity for medical device manufacturers. Vascular access catheters, for example, are used in a variety of medical procedures to access a patient’s blood vessels for many purposes. Some examples include:
1. Central Venous Catheters (CVCs): These types of catheters are inserted into a large vein, typically in the neck, chest, or groin area. They are used for administering medications, fluids, blood products and for collecting samples for diagnostic tests. An example procedure using CVC is chemotherapy administration where powerful drugs are delivered directly into the bloodstream of cancer patients.
2. Peripherally Inserted Central Catheters (PICCs): PICCs are used when intravenous treatments are needed over a long period of time, such as antibiotics administration or total parenteral nutrition (TPN) which provides nutrition through an IV solution when a patient can’t consume food orally.
3. Dialysis Catheters: For patients suffering from kidney failure, dialysis catheters allow for removal and return of blood during dialysis treatment. This type of catheter is designed to withstand high flow rates necessary for the hemodialysis process.
4. Arterial Catheters: These catheters provide access to arterial blood vessels instead of veins and are primarily used for regular monitoring of blood pressure and obtaining arterial blood samples in critically ill patients in ICU settings.
5. Interventional Radiology Catheters: In procedures like angioplasty or stent placement, these specific catheters help doctors see blockages or narrowing inside arteries by injecting contrast dye which can be seen on X-rays.
6. Implantable Ports: A port is small reservoir that is surgically implanted under the skin, usually in upper chest area. It connects to a vein via a catheter. These offer an alternative to frequent needle sticks during therapies that require regular venous access like chemotherapy.
Each device has specific functions and is tailored towards particular treatments depending on the duration and nature of therapy required by the patient.
Despite the importance of these devices in the medical industry, developing new and improved devices is a complex and challenging endeavor.
Product development for these tools and devices is a multi-faceted project. As a result, development projects must incorporate market or healthcare professional’s feedback, field observations, considerations, and principles from a variety of disciplines, such as engineering, biochemistry, and materials science.
Safety and Effectiveness
First and foremost, engineers must be aware that in the medical context, the product is not simply a piece of technology, but the lifeblood of the patient. It is therefore essential that any medical device developed is safe and effective. Additionally, it must perform its intended function with a high degree of reliability and accuracy.
Thus, it is important to ensure the device is compatible with the patient’s body and environment. The compatibility of vascular access products depends on several factors:
1. Patient’s Medical Condition: The specific medical condition or treatment requirement of the patient plays a major part in deciding the suitability of a vascular access product. For instance, patients with chronic kidney disease may require dialysis catheters for regular hemodialysis.
2. Duration of Treatment: The anticipated duration of the therapy is another significant factor. Short term therapies might utilize peripheral IV catheters while long term treatments often require more durable options such as PICCs or implantable ports.
3. Material Compatibility: The material of the catheter should be compatible with the drugs or solutions being administered to prevent reactions and ensure safety and effectiveness. Some medications or solutions can cause damage to certain types of materials, leading to complications like catheter occlusion.
4. Patient Comfort and Lifestyle: The patient’s comfort, lifestyle, and ability to care for the device at home also affects the choice of vascular access product. For example, active patients might prefer an implanted port which can be concealed under skin and does not restrict mobility.
5. Vascular Anatomy: The individual’s vessel size, quality, and location have a direct impact on what type of access device can be used. Conditions like deep vein thrombosis could limit options for central venous catheter placement.
6. Risk Factors for Complications: Certain devices have higher risks for complications such as infections (like CLABSI – Central Line Associated Blood Stream Infection) or thrombosis; these risks must be weighed against their benefits when choosing an appropriate device.
7. Ease of Insertion: Some devices are easier to insert than others; this can influence choice particularly in emergency situations where rapid vascular access is needed.
Understanding these factors helps clinicians make informed decisions about which type of vascular access is most appropriate for each patient’s specific circumstances.
Requirements vs. Design Constraints
One of the primary challenges of vascular access product development is managing the trade-off between design constraints and requirements. The medical device must be designed with a specific set of requirements in mind, such as size, shape, materials, and cost. However, often times these requirements go against one another, and a balance must be struck to ensure the device performs its function without sacrificing one quality for another.
Medical Device Regulations
The regulatory environment is a key factor in vascular access product development. Regulatory agencies must approve any medical device for its intended use before it can be used on or in humans, and regulatory agencies may require additional testing or additional documentation which can add cost and time to the development process. These requirements can include:
1. Preclinical Testing: Laboratory experiments using cells and animal models are carried out to determine the device’s safety and functionality.
2. Clinical Trials: If preclinical testing is successful, human trials commence to evaluate real-world efficacy and safety.
3. Quality System Regulation (QSR): Regulatory entities like FDA mandate compliance with QSR, which encompasses all facets related to a finished medical device’s lifecycle.
4. Risk Analysis Documentation: Comprehensive reports outlining potential hazards associated with product use and mitigation strategies based on standards like ISO 14971 are required.
5. Performance Testing Data: Documents must include performance test data demonstrating that the device operates as intended under different conditions.
6. Biocompatibility Testing Reports: Devices in contact with body tissues or fluids undergo biocompatibility testing to prevent unacceptable biological responses.
7. Sterilization & Shelf-Life Validation: Sterile devices require proof of sterilization method validation and established shelf-life until expiry date.
8. Labeling and Instruction Manuals: Proposed labels, including usage instructions and warnings, need review for accuracy and clarity.
Customer Needs
Finally, vascular access product development requires an understanding of the customer’s needs. Market research must be conducted to understand the patient’s requirements and preferences, and products must be designed to meet those requirements. Let’s take the example of a patient who requires long-term intravenous therapy. Some considerations would be:
1. Comfort: The patient may prefer a catheter that is comfortable and does not restrict their daily activities. For instance, an implanted port allows for more mobility compared to an external catheter.
2. Low Maintenance: As they will be using the product over a significant period, the patient would likely prefer a vascular access device that requires minimal care and cleaning to reduce chances of infection and simplify their routine.
3. Ease of Use: If the therapy involves self-administration of medication at home, ease of use can be crucial. A product design that facilitates easy connection and disconnection of medication lines would be preferred.
4. Minimal Pain or Discomfort: Patients would undoubtedly favor devices that cause minimal pain or discomfort during insertion, use, or removal. For example, PICCs are often considered less invasive than other types of central venous catheters.
5. Aesthetics: Some patients might prefer devices like implanted ports which are concealed beneath skin, making them less visible and thus having lesser impact on the patient’s appearance.
6. Cost-effective: While insurance often covers medical devices, out-of-pocket costs can still be a consideration for many patients.
Through market research and direct feedback from users (patients), healthcare professionals, and caregivers, manufacturers can gain insights about these preferences, which can guide development of new products, or improvement upon existing ones, to meet user needs effectively.
Then, once developed, the medical product must be tested to ensure it meets the customer’s criteria while remaining safe and reliable.
Vascular Access Product Development Process
The development of a vascular access device begins with the initial concept and ends with the delivery of a finalized product. This process can be broken down into various steps, including design, prototype development, testing, and manufacturing.
Catheter Design
The development of a vascular access product focuses mostly on the design of catheters. This vascular access device is typically a tube or catheter inserted directly into a patient’s veins for the purpose of delivering medications, fluids, or nutrition.
Catheter design is a critical part of vascular access product development, as the purpose of the catheter is to access the patient’s veins without causing damage or discomfort. Design considerations are based on factors such as length, diameter, shape, material, and surface texture. These factors can influence how easily the catheter can be inserted, the amount of discomfort caused by the insertion, and the likelihood of a successful procedure.
Prototyping and Testing
During the design phase, engineers and designers create prints and models for the vascular device or component, incorporating the clinical requirements and features specified by the customer. The prints and models are used to produce prototypes for review. Data gathered from the review is analyzed and may result in design modifications.
Once the design is finalized, the prototype will be subjected to a series of tests to ensure it meets safety and performance standards. This includes conducting clinical studies and testing the prototype for parameters like tensile strength, pressure, and flow testing. Once the device has passed all tests and been approved, it is ready for manufacture.
Production Manufacturing Process
Once the prototype is approved, the product is ready for production.
During the manufacturing process, the vascular access device is produced using a variety of processes and equipment, injection molding, extrusion, overmolding, tipping, skiving, printing, assembly, testing, and packaging.
All steps in the manufacturing process must go through a rigorous validation protocol. Validation protocols are developed in collaboration between the customer and the CMO’s quality team. Protocols are designed to ensure a consistent and reliable output that meets the products specification at each manufacturing step.
Only after the validation protocol has been executed and the results approved is the product released for production. Throughout the manufacturing process, quality assurance teams ensure that the vascular access catheter meets the highest standards of safety and reliability. The completed products are then packaged and shipped to the customer, ready for use.
Custom Medical Device Manufacturing
These days, there is a growing trend in the medical industry towards the use of custom medical devices to ensure better patient outcomes and a more personalized approach to treatment.
At Flexan, we take pride in our 30-year history of producing quality products for the vascular access markets. During that time, we have played a significant role working with our customers to help them bring over 40 access catheters to the market. These products have had a profound positive impact on the health outcomes of millions of patients. From the choice of material used in the device to the size and positioning, we work with healthcare professionals and medtech companies to manufacture custom vascular access devices that meet individual needs.
Need Help With Vascular Access Product Development?
For over three decades, Flexan has been at the forefront of medical device manufacturing, and with our expertise, we can help you manufacture your vascular access product and help with its development. Our vascular access device portfolio includes a range of catheters and associated needle-free technology.
By leveraging the latest in catheter technologies we empower healthcare professionals to establish safe, dependable, and efficient access to the vasculature. At Flexan, our advanced technology and services are tailored to meet the unique requirements of vascular access products.
Don’t miss out on the opportunity to elevate your vascular access products. Contact Flexan today to learn how our quality catheters can help you stay ahead in the medtech industry. Reach out now to learn more!
View website








