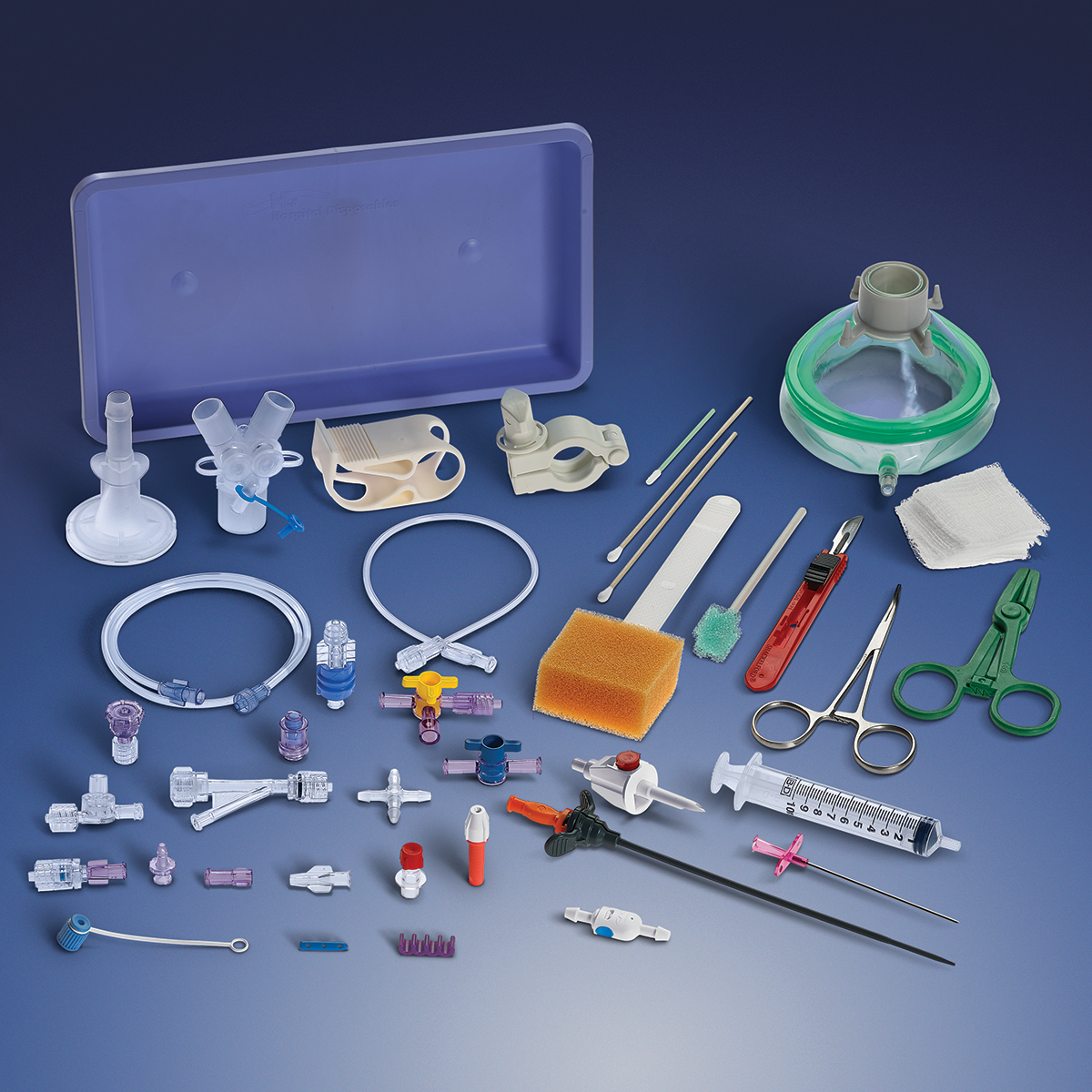
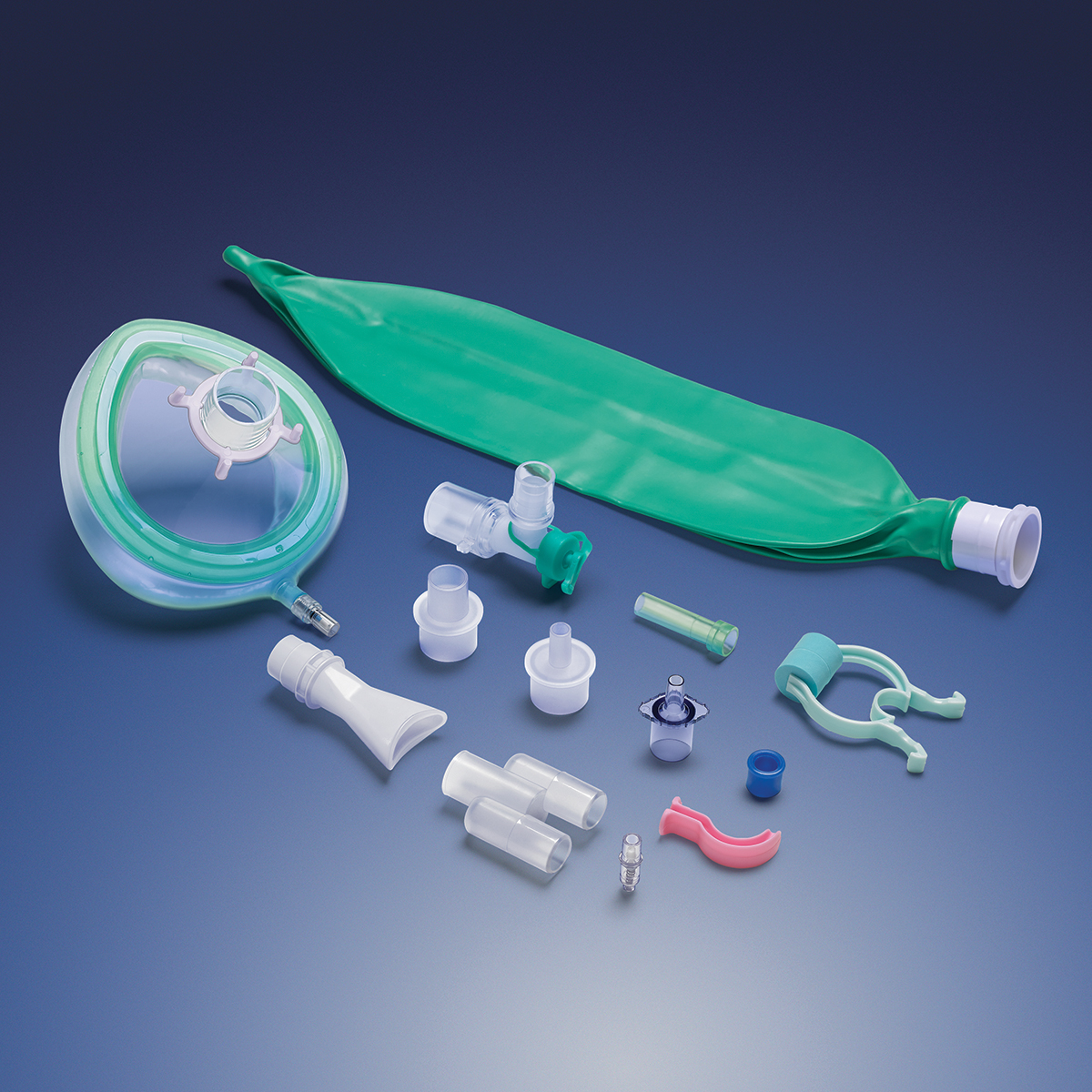
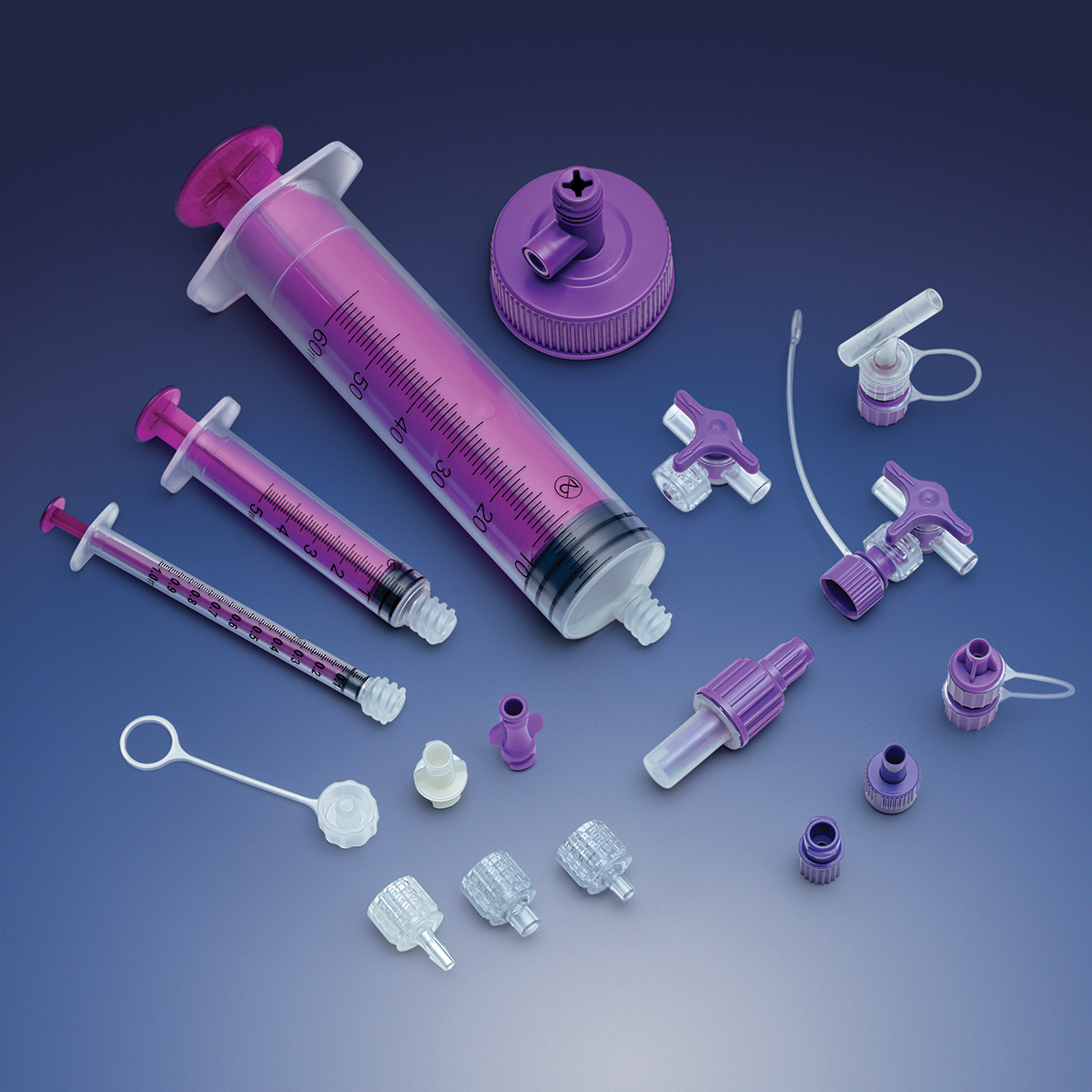
In these uncertain times, Qosina understands it is imperative that its customers quickly adapt to the changing needs of the medical device industry. Since the beginning of the COVID-19 crisis, Qosina has been committed to being a one-stop source for critical medical components.
Qosina carries a broad product line and supplies components that can be used in a wide variety of devices, including fluid delivery, ICU equipment and enteral feeding.
Ronkonkoma, NY, USA, May 5, 2020— In these uncertain times, Qosina understands it is imperative that its customers quickly adapt to the changing needs of the medical device industry. Since the beginning of the COVID-19 crisis, Qosina has been committed to being a one-stop source for critical medical components.
Qosina carries a broad product line and supplies components that can be used in a wide variety of devices, including fluid delivery, ICU equipment and enteral feeding.
Recently, the FDA issued a series of guidance documents that provide policies to help expand the availability of devices to address the COVID-19 public health emergency. This allows more flexibility for manufacturers to make device modifications that cover manufacturing limitations or supply shortages related to COVID-19 without prior approval.
Qosina is happy to be a second source for components and will help customers find alternative products for those that are already approved. Customers can browse qosina.com for critical products to be used in their device manufacturing projects, and can access material and technical documentation, plus 3D CAD models for quick product qualification.
Founded in 1980, Qosina is a leading global supplier of OEM single-use components to the medical and pharmaceutical industries. Qosina’s philosophy is to address its customers’ need to reduce time to market by providing thousands of stock components. The company’s vast catalog features more than 5,000 products shown in full-scale illustrations on a one-centimeter grid. Qosina offers free samples of most items, low minimum order requirements, just-in-time delivery, modification of existing molds, and new product design and development. Qosina is ISO 13485, ISO 9001, ISO 22301 and ISO 14001 certified, and operates in a 95,000 square-foot facility with an ISO Class 8 Clean Room.
To learn about Qosina’s full component offering, which includes the newest products, visit www.qosina.com or call +1 (631) 242-3000. Visit Qosmedix, Qosina’s cosmetics division, at www.qosmedix.com. Qosmedix is a certified global supplier of beauty tools and accessories to the cosmetic, skincare, spa and salon industries.
###
Contact:
Qosina Corp.
Rachelle Morrow
+1 (631) 242-3000
[email protected]
View website