The Institute for Pediatric Innovation (IPI) and Pfizer have partnered to award two $50,000 grants to medical companies for the development of a device to dispense potentially life-saving medicines for children in low-resource settings. Pfizer has developed a new formulation for pediatric drug administration, and together with IPI held a challenge for companies from around the country to design a dispensing device for pediatric drug formulation.
Boston, Massachusetts (PRWEB) December 15, 2016
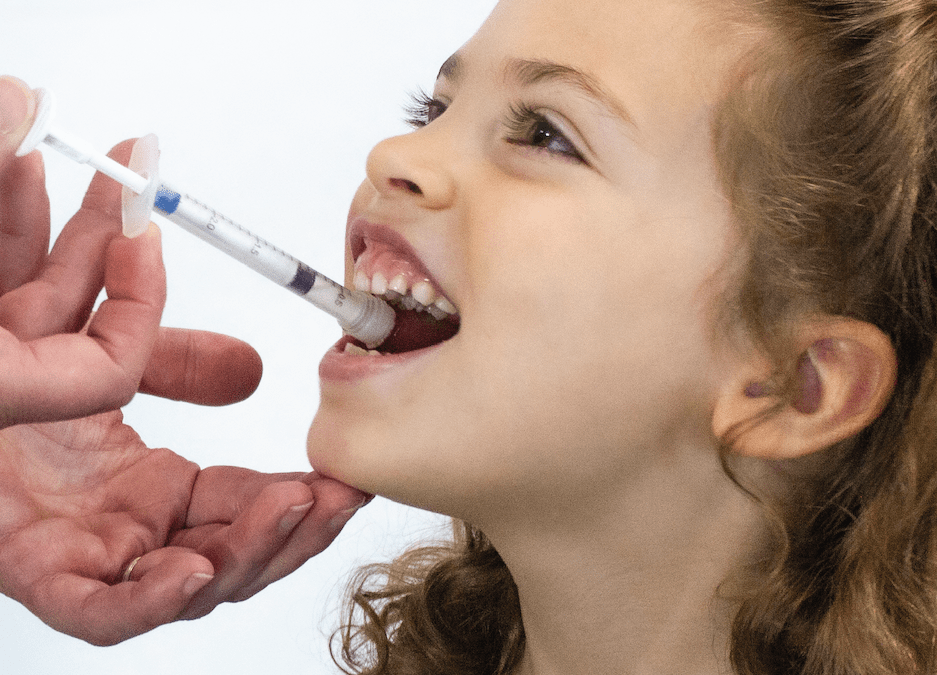
The Institute of Pediatric Innovation (“IPI”) has partnered with Pfizer Inc. (“Pfizer”)(NYSE: PFE) in an open innovation challenge to solicit and support innovative ideas for a system consisting of a package and dispensing device that will be used to deliver oral solid multiparticulate (MP) drug-formulated medicines to children.
The challenge attracted 25 submissions. A panel led by IPI, composed of engineers, end users, and experts from the Gates Foundation, New England Pediatric Device Consortium, PATH, and Pfizer, reviewed submissions to decide which proposals would receive funding. Proposals were evaluated on four main criteria: dose accuracy, cost, end user ease of use, and cultural appropriateness.
Upon review the panel awarded grants of $50,000 each, to two applicants: Balda C. Brewer, Inc and HS Design (‘HSD’)
While many of IPI’s projects are focused on pediatric healthcare in the United States, it sees this collaboration as a historic opportunity to branch out into pediatric global health. Pfizer has developed the state-of-the-art solid MP drug reformulation technology. This technology addresses taste, storage, and other factors essential for safe, accurate, and adherent administration, making it ideal for administering medicines to children in low-resource settings.
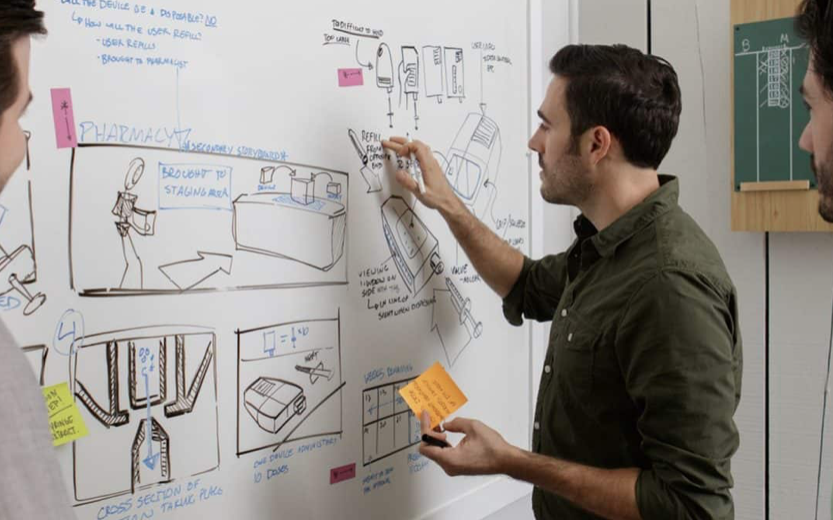
One winning product was designed by Balda C. Brewer, Inc., of the Balda Group (“Balda”). Balda has extensive experience creating plastic solutions for the healthcare industry, from syringes to handheld blood-glucose level testing devices. Their product design was based on a sluice gate. It features a flexible plastic tube with a mechanical actuator that measures the doses and dispenses the multiparticulates. The system can be injection molded and is compatible with standard storage containers.
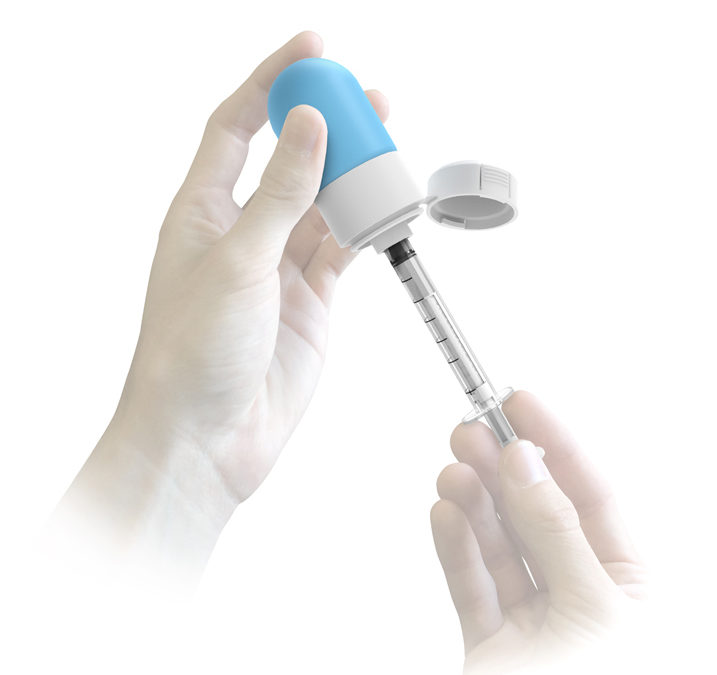
HS Design (“HSD”) submitted the other winning design. HSD, an ISO 13485 certified product development firm, is a recognized leader in user centered medical design. HSD has been providing successful design and development services for over 40 years with extensive understanding of the ethics and regulatory nature of the industry. This expertise has led to a portfolio of over 400 successfully designed surgical tools, diagnostic instruments, consumer healthcare products and delivery systems. HSD’s novel design features a collapsible bag in which to store the multiparticulates and a syringe for dosing. This system is designed to be used similar to existing liquid oral syringes and vials, which minimizes the time required to learn to use the product properly. HSD has partnered with a global contract manufacturer to fully develop the delivery system.
According to IPI president Don Lombardi, “We are pleased to partner with Pfizer and collaborate with our two grantees on formulation and delivery technologies that can help to transform the field of oral medicines for children. We congratulate Balda and HSD for their efforts.”
Richard Korsmeyer, Executive Director of Technology & Innovation at Pfizer, said “We are excited to facilitate creative solutions that address the needs of pediatric patients and are glad to see the interest generated by the Challenge.” IPI and Pfizer congratulate Balda and HSD. IPI will be working with both companies as they advance their concepts to working prototypes over the next 24 months.
Since IPI got its start 10 years ago, it has been committed to creating a world where even the youngest patients have access to the highest quality drugs and medical devices fitted specifically to their needs. In light of this goal, IPI collaborates with children’s hospitals, industry partners, and other stakeholders to catalyze the development of medical devices and drugs and to bring these products to market. For more information, visit http://www.pediatricinnovation.org.