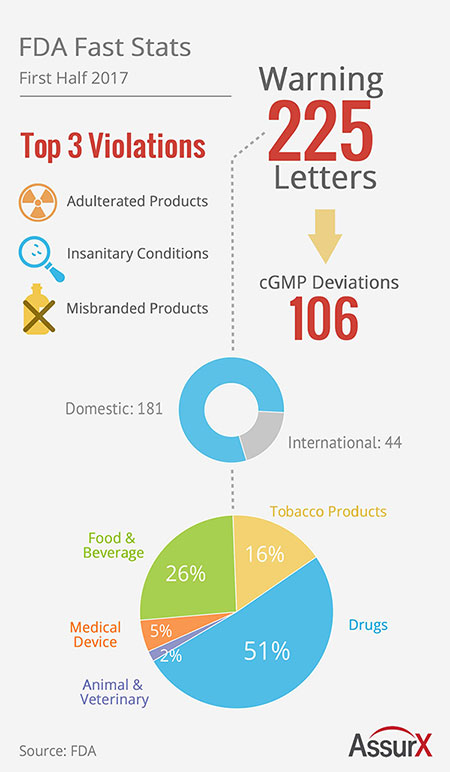
The FDA released 225 FDA warning
letters in the first half of the year. AssurX, a QMS software
provider, provides a quick snapshot detailing the FDA’s activity.
Top
3 FDA Warning Letter Violations
The top 3 FDA Warning Letter violations were (1) adulterated
products, (2) insanitary conditions and (3) misbranded products. All
three violations were applicable to both drugs and food.
Number of FDA Warning Letters
In the first half 2017 a total of 225 FDA Warning Letters were released. 181 domestic manufacturers received FDA Warning Letters while 44 international manufacturers received FDA Warning Letters. This correlates to approximately 80% of FDA Warning Letters issued to domestic manufacturers while 20% of the FDA Warning Letters issued by inspectors were to international manufacturers.
Also of note were 106 cGMP deviations recorded.
Manufacturer Type Breakdown
The breakdown of manufacturer type receiving FDA Warning Letters:
- 51% Drug Manufacturers
- 26% Food & Beverage Manufacturers
- 16% Tobacco Product Manufacturers
- 5% Medical Device Manufacturers
- 2% Animal & Veterinary Product Manufacturers
Key Insights
- The FDA continues to follow complaints across all manufacturing entities and is clearly taking those complaints seriously.
- Drugs including API and supplement manufacturers received more than half of all warning letters.
- Supplement manufacturers have received a slew of letters for unvalidated scientific claims about the healing properties of their “natural” product ingredients.
- The vaping/e-cigarette industry is now feeling the pain of being subject to new rules added August 8, 2016 to the Tobacco Control Act.
- Insanitary seafood preparation and packing still remain a major HACCP violation.
Conclusion
In calendar year 2016, the overall number of quality management system surveillance inspections by the FDA was slightly higher. Certain citation patterns are certainly emerging in 2017. Will the 80/20 warning letter breakdown between domestic and international manufacturers continue for the rest of 2017?
AssurX
With decades of expertise built into our quality management and regulatory compliance software platform, AssurX helps medical device manufacturers maintain quality and FDA compliance, streamline workflow, control risk and better manage any enterprise. Our incredibly configurable software and deep understanding of users’ needs produce a unique system that easily adapts as your business evolves. AssurX is an ideal partner for FDA regulated companies looking for better operational control and efficiency while staying compliant.
To learn more about AssurX visit www.assurx.com.
For additional insights on quality management and compliance related issues impacting the medical device industry visit www.assurx.com/blog.